Major Ions
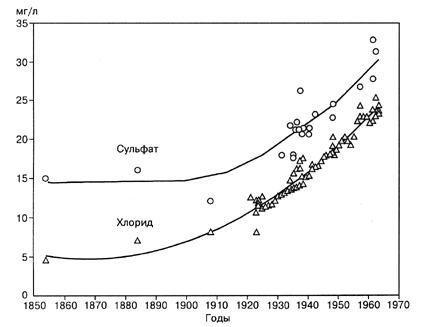
Water of Lake Baikal is weakly mineralized. The total concentration of salt is ca. 100 mg/1. In order to understand, if any changes in the ecological system of Lake Baikal occurred since industrial revolution in Siberia, i.e. since the middle of the 1930-es, it is interesting to find out whether there have been any changes in the concentrations of major ions. Such a change has occurred in some large lakes. Fig. 2.1.1 shows the dynamics of the concentrations of the sulphate anion in one of Great Lakes of America -Lake Ontario - during the time interval between the middle of the XIX century and 1963 (Beeton, 1970). It is seen that this concentration increased approximately twice, from 15 to 30 mg/l. It will be mentioned that Lake Ontario is one of the most polluted of the five Great Lakes.
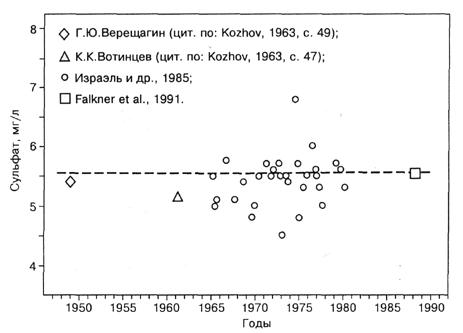
Fig. 2.1.2 presents data of different authors on the concentrations of sulphate anion in Lake Baikal. It is seen that data obtained in 1965 - 1981 within a governmental program of monitoring of Lake Baikal (Izrael et al, 1985) are highly scattered, from 4 to 7 mg/1. Anyhow, they, together with those obtained by other workers, do not reveal any reliable long-term trend. As for the scatter, it is undoubtedly due to error of measurements, rather than due to any changes in the ecological system of Lake Baikal. In principle, one could imagine, that large amounts of sulphate were discharged into Lake Baikal in certain years, and this resulted in an increase of the concentrations, but there is no way to explain, how dozens of million tons of sulphate - a conservative ion - could disappear from the ecological system of Lake Baikal during a period of less than one year (cf. Fig. 2.1.2 and Table 1.1 in the Introduction ).
An international expedition, one of the tasks of which was to measure the concentrations of major ions in Lake Baikal at different locations and depths, took place in 1988. Measurements were done by means of modern instruments and methods. Chloride and anions were determined by means of ion chromatography an accuracy of ± 5%; Ca, Mg, Na, K cations - by means of atomic absorption spectrophotometry with accuracy of 1, 1, 3 and 2%, respectively. The results (Falkner et al, 1991) are shown in Fig. 2.1.3. It is seen that the concentrations of major ions are the same in the three basins of Lake Baikal at all depths.
Years
Fig. 2.1.1. Increase in the concentration of sulphates in Lake Ontario (USA) since the industrial revolution (Beeton, 1970).
Years
Fig. 2.1.2. Concentration of sulphate in Lake Baikal reported by different authors. G.Yu. Vereshchagin and K.K. Votintsev used the method described by G.Yu. Vereshchagin (1933).
It is interesting that the concentration of sulphate found by means of modern techniques equal to 5.5 mg/1 is close to that obtained by G. Yu. Vereshchagin in the 1930-40ies; the meanconcentration of sulphate for all depths of Southern Baikal at that time was found to be equal to 5.33 mg/1 (cited according to Kozhov, 1963, p. 49). The fact that the data of G.Yu. Vereshchagin is close to those of Falkner et al. (1991) is one more argument which confirms that the scatter of the data of 1965 - 81 in Fig. 2.1.2 is due to errors of analysis. It is evident that the techniques employed were not suitable for monitoring of sulphate in Lake Baikal. It is also evident that wrong are conclusions on the heavy pollution of Lake Baikal with sulphate published by E.Tarasova and A.Meshcheryakova (1992), data and conclusions of G.Galazy >and E.Tarasova (1993) «On the Background Content of Sulphates in Lake Baikal» who claimed that the concentrations increased within the time interval since 1967 from 3.9 to 6.5 mg/1. Unfortunately, uncorrect is also the conclusion of K.K.Votintsev and I.B.Mizandrontsev (1981) who claimed that the mean concentration of sulphate in 1950-1975 was 4.85 mg/1. Inefficient accuracy is illustrated by the fact that the 95% confidence interval of this series of data spans an interval from 4.42 to 6.28 mg/1 corresponding to inventories varying between 110 and 157 mln tons. Data of Falkner et al. (1991, 1997) suggest that the data published by I.Lomonosov et al. (1989) and Yu.Fedorov (1993) are also wrong. For example, Lomonosov etal. (1989) reported that the concentrations of sulphate in Southern Baikal near the site of discharge of waste waters of the Baikalsk Pulp and Paper Plant in different years and months vary from 1.4 to 29.6 mg/1. The concentration of sulphate in the waste waters is high (100-200 mg/1), and it is not surprising that increased concentrations of the anion are found near the outlet. However, it is very hard to imagine how the plant could make water contain four times less sulphate than background at the same point.
The fact that anthropogenic impact has not yet changed the concentrations of major ions in waters of Lake Baikal is illustrated by the finding (Falkner et al., 1991) that they are the same in the three basins of Lake Baikal at all depths (Fig. 2.1.3.) - if any significant changes would have happened due to anthropogenic impacts, they could not be the same in different basins Fig.2.1.3.
The above considerations do not mean that pollution of Lake Baikal with major ions does not create any danger. On the contrary, ions like sodium, magnesium chloride and sulphate are conservative, i.e., they are not buried in bottom sediments. Hence, it is evident, that development of economic activities in the catchment basin of Lake Baikal leads to slow, but progressive increase in their concentration in the lake. This threat has been long ago noticed by Votintsev (1973). Slow salinization does not create an immediate danger on the condition that waters of Lake Baikal are intensively mixed to the very bottom, like it takes place nowadays. However, recent studies by Klerkx et al. (1993) and Hohmann et al. (1997) reveal that an essential feature of the mechanism of renewal of deep waters in Lake Baikal is its dependence on minor fluctuations in salinity - even small fluctuations in salinity (1-2% of the present) significantly change buoyancy of water parcels, compared to surrounding Baikal water. If, under appropriate conditions, water with increased salinity delivered by tributaries will concentrate near the bottom, it may finally lead to a change in the mechanism of renewal of deep waters, and oxygen-saturated surface waters will not penetrate to the bottom. Formation of an anoxic near-bottom layer would result in mass extinction of endemic benthic organisms.
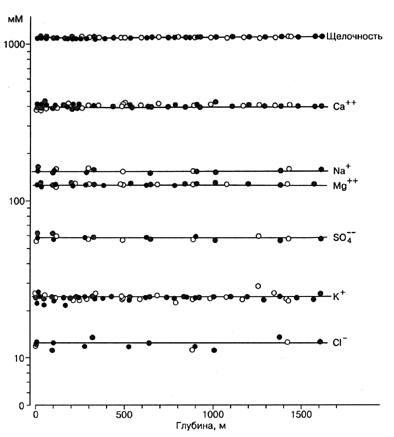
Fig. 2.1.3. Concentration of major ions in Lake Baikal. Gray symbols - Southern,light symbols - Central, dark symbols - Northern basin. Mean concentrations of calcium 16.1, magnesium 3.02, sodium 3.57, potassium 0.94, chloride 0.44, sulphate 5.51 mg/l (ppm). Falkneretal., 1991.
However, before we decide whether this scenario is realistic, we need to perform many studies using precise physical and chemical methods. Such studies are already on the way. In particular, precise measurements of water conductivity were done with oceanographic CTD probes. It was found that the calculated total salinity in all the pelagial of Lake Baikal varies from 94.3 to 95.5 mg/1 (N.Granin, personal communication). Changes of salinity along vertical profiles do not exceed 0.5-0.7 mg/1. This fact once again illustrates that all the earlier published claims on the «patchiness» of major ions in Lake Baikal and on the «trends» of their changes are invalid.



